
Balance the equation AgNO3 + Na2CO3 = Ag2CO3 + NaNO3 using the algebraic method.

Silver nitrate is one of the few soluble silver compounds. When hydrochloric acid (chemical formula: HCl) reacts with sodium carbonate (chemical formula: Na2CO3), one of two reactions will take place, depending on the relative … The balanced net ionic equation for precipitation of CaCO3 when aqueous solutions of Na2CO3 and CaCl2 are mixed is _. The answer will appear below Always use the upper case for the first character in the element name and the lower case for … Second, … by | | Uncategorized | 0 comments. As a result of adding N a2CO3 and AgNO3 products formed are NaNO3 and Ag2CO3. Practice Problems: Double Replacement Reactions → 1. Q: (NH4)2SO4(aq) + Na2CO3= Balanced equation total ionic equation Net ionic equation. NaNO3 is a soluble substance when … In the Fétizon oxidation, silver carbonate on celite serves as an oxidising agent to form lactones from diols. 1/2 mL 0.01 M AgNO3 + 1/2 mL 0.1M Na2CO3 formed a precipitate. Use uppercase for the first character in the element and lowercase for the second character. How many moles of the excess reactant remain after the completion of the reaction? This is called a precipitation. This is the best … The reaction between NaOH and AgNO3 produces NaNO3, Ag2O and H2O. 17 How many moles of precipitate will be formed when 45.4 mL of 0.300 M AgNO3 is reacted with excess Calz in the following chemical reaction? Zoek uit wat voor soort reactie er is opgetreden.

(b) Acid-Base reaction: There is no change in oxidation number for any of the elements in this reaction-hence it is NOT an oxidation- reduction reaction. Make one more intelligence and profound comment. This reply was modified 9 months, 2 … Correct answer to the question For the reaction represented by the equation AgNO3 + NaCl ® NaNO3 + AgCl, how many moles of silver chloride, AgCl, is produced from 7.0 mol of silver nitrate AgNO3? However the AgOH would break down into Ag2O and H2O. A typical reaction with silver nitrate is to suspend a rod of copper in a solution of silver nitrate and leave it for a few hours. What Is The Reaction Prediction For Na2CO3+AgNO3? Who are the experts? Chemical reactions Сhemical tables. While NH3 reacts with Na+, the solution become soluble and there is no chemical changes.
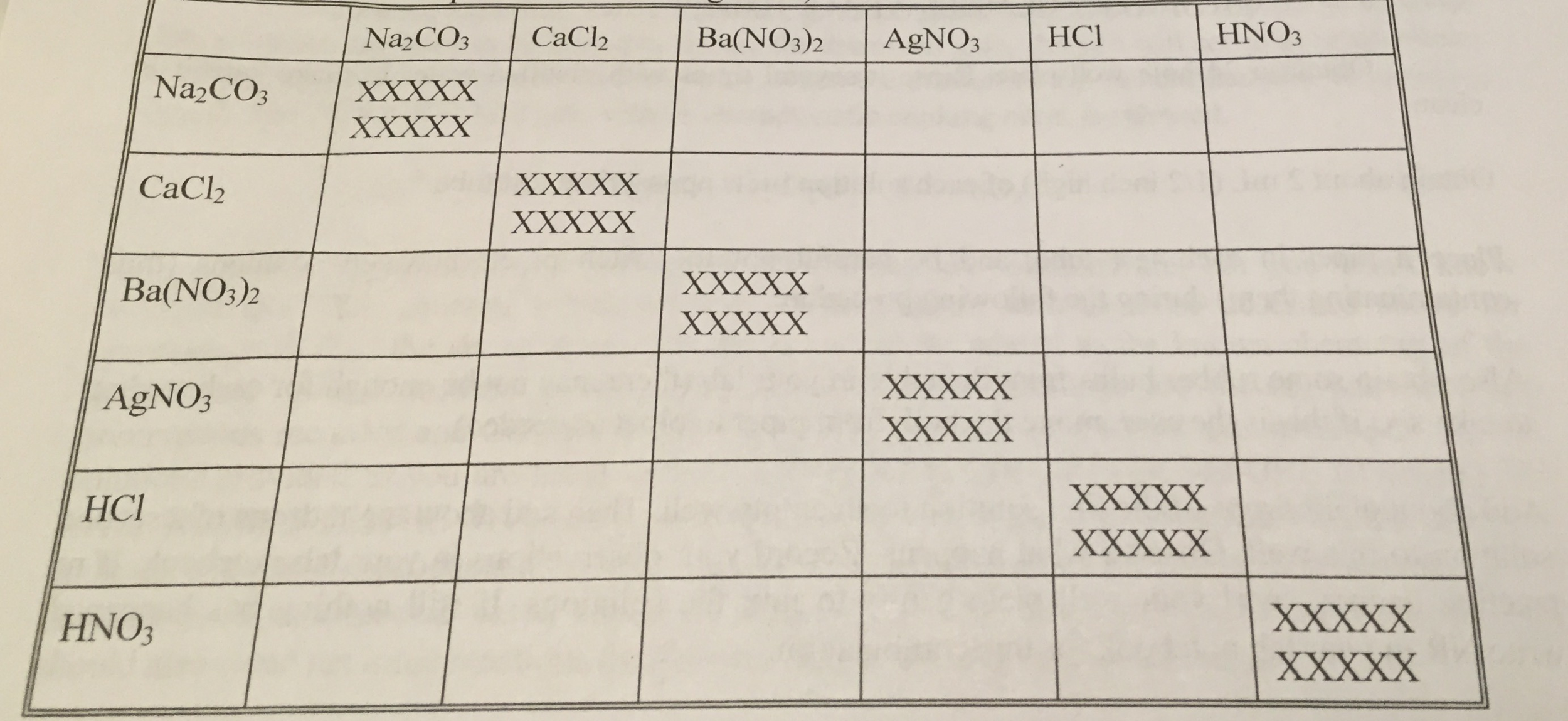
Na 2 CO 3 + AgNO 3 is a … Li2S (aq) + AgNO3 (aq) 2. NaCl(aq) + AgNO_3 (aq) → AgCl(s) + NaNO_3(aq) This is a … After decomposition, the Na2CO3 had a mass of 1.57 grams. 2 AgNO3(aq) + Na2CO3(aq) → Ag2CO3(s) + 2 NaNO3(aq) Since Silver carbonate has a very low solubility in water (0.031 g/L (15 ☌)), it will precipitate as a white solid. one of the products of a chemical reaction is a gas. The reaction is called a precipitation reaction. It might even occur at the microscopic level, which you might be able to see under a microscope. At an atomic level there likely is a reaction. BaCl2 (aq) + Na2CO3 Based on the table given, a reagent that distinguish the chemical properties of Mg2 + and Na+ is NH3. Write a balanced chemical equation for the reaction.
#AGNO3 NA2CO3 PRECIPITATE FULL#
In a full sentence, you can also say AgNO3 (silver nitrate) reacts with Na2SO4 (sodium sulfate) and produce NaNO3 (sodium nitrate) and Ag2SO4 (silver sulfate) Phenomenon after AgNO3 … A little balance and identify. However, when both of them react, sodium ions form ionic bond with nitrate ions while silver ions form bond w. The labeled beakers → NaCl + CaCO 3 + 2NaCl chloride combines with.
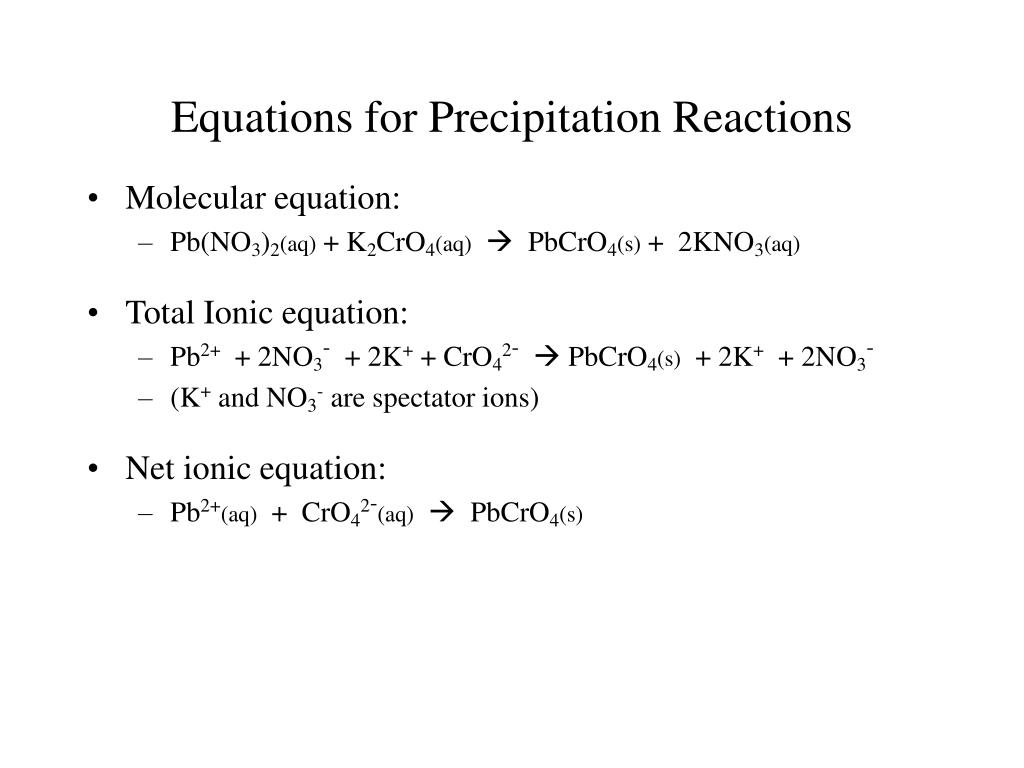
Aqueous silver nitrate will react with aqueous sodium carbonate in a double displacement reaction to produce a silver carbonate precipitate and aqu. T F The oxidation state of nitrogen changes from +6 to +2. 2 AgNO3(aq) + Na2CO3(aq) → Ag2CO3(s) + 2 NaNO3(aq) Since Silver carbonate has a very low solubility in water (0.031 g/L (15 ☌)), it will precipita. It is because when NH3 reacts with Mg2+, the solution become insoluble and turn into white precipitate.


 0 kommentar(er)
0 kommentar(er)
